Solid oxide fuel cell - make electricity from natural gas
关键词:
electrolyte layers、oxide ion、oxide ion conductive electrolyte、solid oxide fuel cell、typical electrolyte material、electrolyte current directors、oxide ion conductors、Cell current、electrolyte lattice、single cells、Manganite complex oxides、Typical electorate materials、oxygen ions、Dockside fuel cells、First oxide powders
文字记录:
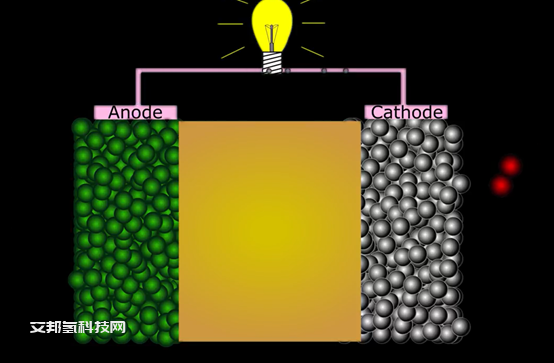
Solid oxide fuel cell is an electrochemical device, which converts chemical energy to electrical energy. It typically operates at temperatures from 600. Up to 950 degrees at these temperatures director light cut out. And then our oxide ion conductors, the fuel cell needs a constant flow of fuel fuel such as hydrogen or methane is directed to the note where it reacts with oxygen ions from electrolyte lattice. Released Electrons are transferred to the cathode through an external load. In the cut out, the oxygen molecule absorbs and dissociates the oxygen atoms in the presence of electrons that served oxygen Atom will be reduced the oxide ion? Doc said I and move through the electorate to the note and direct with hydration from a field.
Electrolyte is ion conductive, ceramic solid oxide membrane electrolyte material should not have electronic conductivity and it has to be tense to stop gas flow through it typical electrolyte material is yttria stabilized, Cercone A. Catherine material has to be good electron an oxide ion conductor. It is where oxygen reduction takes place typical Gotham material is Lanthanum Strontium Cobaltite. Hello material has to be a good catalyst oxide iron as well as electron conductor and it needs to be porous. These properties are achieved. It metallic nickel and oxide ion conductive electrolyte are mixed.
Electro chemical reaction takes place on triple phase boundary activity for note is also largely defined by length of triple paste founder E as well as porosity of electrode one unit cell. In the SOFC Stack contains 2 current collectors with castles channels cathode anode and electrolyte current directors are made from special steel with coatings.
Several technologies are applied for production of SOFC Membrane Electrode Assembly. Is most widely used our technology is based on screen printing and typecasting?
First oxide powders are polarized and thoroughly mixed. Then mix beef organic solvents dispersants plasticizers and pointers obtained based off an out and electorate will be typecasted to get the Finn.
Field. Typical electorate materials are Ultra Stabulas Erchonia scan destabilize aconia and Cadelina doped Siri A. After salute trying of tape cassett layers elimination of layers will be carried out for this.
The layers are vacuum ized in a plastic bag. Do. In this demonstration, we laminate layers using is a static pressing at elevated temperatures. Technically, it's like putting the layers in the seawater approximately 600 meters below sea level. As a result, the cathode and electrolyte layers have stick together. After lamination layers of anode and electrolyte will be centered in high temperatures from 1200 to 1500 degrees. Solid oxide anode electrolyte dissembling will be covered with Cather layer using a screen printing method. Typical gathered materials are Lanthanum Strontium Cobaltite and lanthanum strength. Manganite complex oxides.
After making the cut out layer the heat treatment will be carried out the cat has been applied separately from an old electorate assembly because it needs termite treatment at lower temperatures. If single cells are completed, then they will be stuck together with interconnect materials with cash flow channels. The voltage of a stack can be built up by adding required number of single cells in series.
Here we have assembled a small demo single cell. Methane flame heated up and provides a flow of fuel at the anode of course in real so FC Stack. Fuel is consumed more efficiently. Sorry Dockside fuel cells operate at temperatures from 600 to 900. 50 degrees Celsius maximum voltage obtained was zero point 94 waltz, which is close to the theoretical value endured chambers. Cell current was up to 25000000 purse advantages of solid oxide fuel cells.
Is that no platinum is needed and in addition to hydrogen? It is able to utilize would gas methane or other carbonaceous fuels. Electrical efficiency of solid oxide fuel cell might be approximately up to 70%. Which is also twice deficiency of classical detentions solid oxide fuel cell systems have been developed for the size of small power plan for single household trains boats as well As for cars.
Excess mean energy could be stored in hydrogen and converted back to electricity using SFC the development of SoC other with materials is mainly focused on improvement of sulfur tolerance. And Redux Stability Noah fully oxide based channels are under development for example, Lantern Strontium comb manganite and Lanthanum Strontium Titanate.
Maine directions of cathode development. Are the improvement of oxygen electrode reduction activity and electrode stability against micro concentrations of water and sulfuric compounds in air?
 艾邦氢能产业链通讯录,目前有2200人加入,如亿华通、清极能源、氢蓝时代、雄韬、氢牛、氢璞、爱德曼、氢晨、喜马拉雅、明天氢能、康明斯、新源动力、巴拉德、现代汽车、神力科技、中船712等等,可以按照标签筛选,请点击下方关键词试试
资料下载:
艾邦氢能产业链通讯录,目前有2200人加入,如亿华通、清极能源、氢蓝时代、雄韬、氢牛、氢璞、爱德曼、氢晨、喜马拉雅、明天氢能、康明斯、新源动力、巴拉德、现代汽车、神力科技、中船712等等,可以按照标签筛选,请点击下方关键词试试
资料下载:

